Why Ice Floats: The Science Behind This Fascinating Phenomenon
Written on
Chapter 1: Understanding Ice and Its Unique Properties
Ice floating on water might seem unusual, but this peculiarity underscores the fascinating characteristics of water, which is essential for life. Despite appearing ordinary, water has some rather odd properties when compared to most other substances.
As a child in the 1990s, I was captivated by lava lamps—those mesmerizing displays of colored wax moving up and down in water. They remain an intriguing example of how different liquids can interact when heated.

By Dean Hochman from Overland Park, Kansas, U.S. — lava lamps, CC BY 2.0.
Lava lamps illustrate the behavior of two immiscible liquids when heated. The lamp contains a waxy substance and water, heated by a light bulb at the bottom. As the wax heats up, it becomes less dense and rises. This occurs because heating causes the particles within the wax to move apart, reducing its density. Once the wax reaches the top and cools, its density increases, causing it to sink again, perpetuating this mesmerizing cycle.
But why does water behave differently? While the wax in a lava lamp sinks as it cools, ice floats because it is less dense than liquid water.
Section 1.1: The Molecular Structure of Water
This anomaly is rooted in the molecular configuration of ice, which I previously discussed in my article about snowflakes. Water is a polar molecule, allowing its molecules to form weak bonds known as hydrogen bonds.
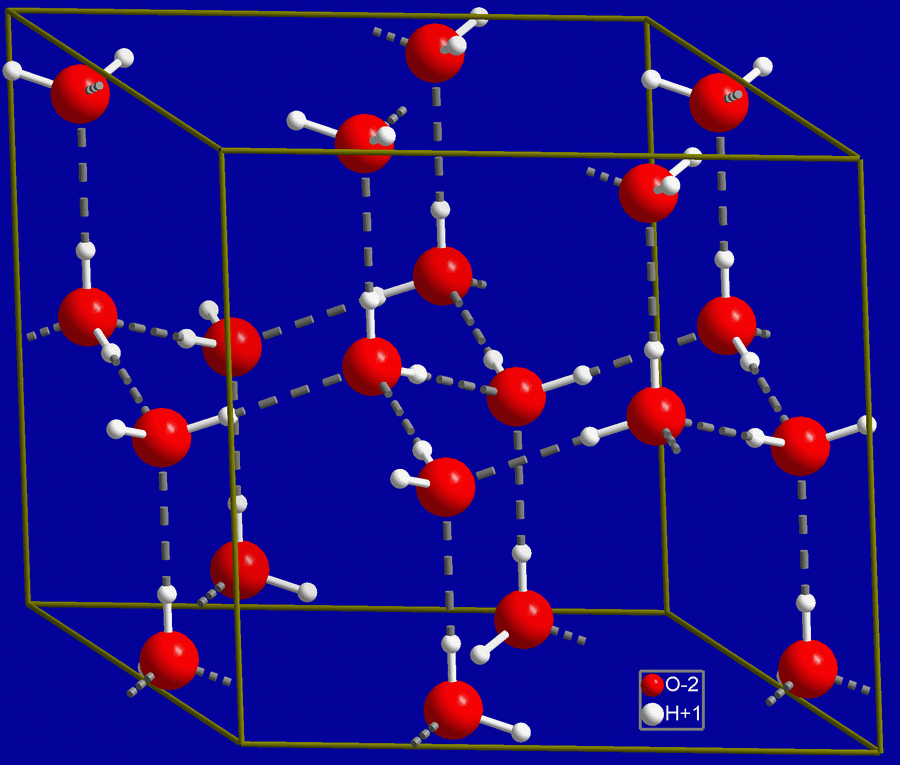
Hydrogen bonds, while weaker than covalent or ionic bonds, are relatively strong compared to other intermolecular forces. In liquid water, molecules are in constant motion, creating a dynamic environment where hydrogen bonds are present but not fixed. When water freezes, the molecules slow down and arrange into a structured lattice, resulting in the formation of ice. This arrangement keeps the molecules spaced apart, making ice less dense than its liquid counterpart, and thus it floats.
Section 1.2: The Importance of Ice Floating
The significance of ice floating cannot be overstated. It plays a crucial role in sustaining aquatic ecosystems. During winter, ice forms a protective layer over lakes, insulating the water beneath. This insulation is vital for aquatic life, as it prevents the entire body of water from freezing solid.

Without this insulating ice layer, many aquatic habitats would be unable to support life during frigid temperatures. The high heat capacity of ice means that it takes a considerable amount of time for the underlying water to warm up again, preventing abrupt temperature changes that could be detrimental to marine organisms.
Chapter 2: Conclusion on Water's Unique Behavior
In summary, water's unusual properties, especially why ice floats, are essential for life on Earth. As we navigate through cycles of ice ages, understanding this phenomenon deepens our appreciation for the natural world.
To explore further about my thoughts and writings, feel free to check my profile linked below:
The first video titled "Why Does Ice Float? Why is that Amazing?" offers a captivating overview of this phenomenon and its implications.
The second video, "Why does ice float by Doctor C," provides an engaging explanation of the science behind why ice is less dense than water.