Understanding the Mechanisms Behind Chronic Fatigue Syndrome and Long Covid
Written on
In 1955, an outbreak of encephalomyelitis occurred at the Royal Free Hospital Group in London, U.K., resulting in the hospitalization of over 200 individuals. This condition refers to inflammation of the brain and spinal cord, usually caused by infections. Interestingly, around 2% of these patients later developed what is now recognized as myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS), which is also considered a subtype of long Covid.
This event was documented in the British Medical Journal in 1957, marking one of the earliest accounts of ME/CFS. Yet, the following years were characterized by widespread skepticism regarding this illness. Some experts even attributed ME/CFS to psychosocial factors, viewing it as a form of hysteria. Consequently, research efforts into ME/CFS were severely limited, and securing funding for studies on this condition proved notoriously difficult.
Today, the repercussions of this neglect are evident. The underlying mechanisms of ME/CFS, along with its risk factors, prognosis, and treatment options, remain poorly understood. While various studies have explored different aspects of ME/CFS, none have provided a unifying and convincing explanation.
However, this situation is poised to change thanks to recent comprehensive research conducted by the U.S. National Institute of Health (NIH).
A Comprehensive Exploration
The NIH study, titled Deep phenotyping of post-infectious myalgic encephalomyelitis/chronic fatigue syndrome, was published in Nature Communications in February 2024. The paper boasts over 70 authors, reflecting the extensive research involved.
From the outset, the NIH study effectively recruited appropriate participants. Given the lack of a definitive diagnostic biomarker and the existence of more than 20 diagnostic criteria, previous ME/CFS studies faced recruitment challenges. To mitigate this issue, the NIH implemented stringent inclusion criteria, which involved thorough medical and psychological evaluations to minimize the chances of misdiagnosis.
As a result, the study identified 17 ME/CFS patients from an initial pool of 217 potential cases during the recruitment phase spanning from 2016 to 2020. Remarkably, one-fifth of the original cases were ultimately attributed to other medical conditions.
The 17 patients selected exhibited similar symptom manifestations, durations, and triggers (usually some form of infection). They were matched with 21 healthy controls based on age, sex, and body mass index (BMI).
Initially, one might question the significance of such a small sample size. However, it became clear that the consistency and accuracy of this group took precedence over sheer numbers—quality over quantity—especially given the intricate nature of the pathomechanisms involved. It is crucial to ensure that the group accurately reflects the condition being studied.
Participants underwent a variety of medical assessments to map out the physiological processes occurring within their bodies. The resulting paper spans 24 pages, with nearly 10 pages dedicated to methods alone, indicating the depth of the research.
The findings revealed that ME/CFS is associated with:
Cardiovascular Autonomic Dysfunction
Compared to healthy individuals, ME/CFS patients demonstrated decreased heart rate variability (HRV), characterized by an elevated daytime heart rate (indicating increased sympathetic activity) and a lesser reduction in nighttime heart rate (indicating decreased parasympathetic activity), along with prolonged blood pressure recovery times (suggesting compromised baroreflex-cardiovagal function).
The autonomic nervous system regulates involuntary functions, including heart rate. HRV measures the variation in time between heartbeats, serving as an indicator of the heart's capacity to adapt to various physiological states and stressors. Higher HRV typically reflects better cardiovascular fitness and resilience to stress, while lower HRV may indicate stress, fatigue, or underlying health issues.
The baroreflex-cardiovagal function pertains to the vagus nerve's role in managing heart rate in response to blood pressure fluctuations. When blood pressure rises, the baroreflex activates the vagus nerve to slow the heart rate, stabilizing blood pressure. Conversely, when blood pressure decreases, this reflex reduces vagal tone, allowing heart rates to rise.
The combination of lower HRV and diminished baroreflex-cardiovagal function in ME/CFS patients suggests a compromised autonomic nervous system control over cardiovascular function.
Impaired Brain-Muscle Connection
Both healthy controls and ME/CFS patients exhibited similar maximum grip strength. However, ME/CFS patients struggled to sustain this strength for as long or with as many repetitions as their healthy counterparts. This indicates that fatigue in ME/CFS is not rooted in muscle weakness but rather in neuromuscular control.
Subsequent brain stimulation tests revealed that the motor cortex in healthy individuals returned to baseline activity after exercise, while it remained active in ME/CFS patients. This implies that their brains remain overly engaged and potentially less effective in managing muscle movements.
Further brain imaging studies indicated decreased activity in areas responsible for sensory integration and motor response in ME/CFS patients, such as the temporoparietal junction, superior parietal lobe, and right temporal gyrus, which also influences the motor cortex's function.
These differences in brain activity suggest that ME/CFS patients struggle to maintain brain-muscle engagement during extended or repetitive tasks.
Reduced Cardiopulmonary Performance
During cardiopulmonary exercise testing, both groups reached similar peak respiratory exchange ratios, indicating maximal effort. However, ME/CFS patients displayed lower peak power, respiratory rates, heart rates, and oxygen uptake.
This implies that while ME/CFS patients can exert maximum effort, their physical output does not align with their exertion levels, revealing a gap between effort and actual performance.
Additionally, ME/CFS patients exhibited a slower heart rate increase during exercise and reached their anaerobic threshold—where the body transitions from aerobic to anaerobic metabolism—at lower oxygen consumption levels, suggesting an earlier onset of fatigue.
This shift to anaerobic energy production indicates an inability to meet energy demands through oxygen-based processes alone, occurring at lower exercise intensities and suggesting compromised cardiopulmonary fitness in ME/CFS patients.
Altered Neurotransmitter Activities
In the cerebrospinal fluid of ME/CFS patients, levels of DOPA, DOPAC, and DHPG were found to be lower compared to healthy controls. DOPA is a precursor for dopamine synthesis, while DOPAC and DHPG are metabolites of dopamine and norepinephrine.
Interestingly, the levels of dopamine and norepinephrine themselves did not differ between the two groups. These neurotransmitters belong to a category called catecholamines. Thus, changes in their precursors or metabolites indicate issues in the processing or regulation of these neurotransmitters. Altered catecholamine levels were also linked to physical and cognitive fatigue.
Further analyses revealed significant alterations in metabolites associated with the tryptophan and serotonin pathways in ME/CFS patients, especially in females. In contrast, specific decreases in threonine and glutamine were noted in male patients.
The cerebrospinal fluid profile provides a more accurate reflection of brain activity compared to standard blood tests. Changes in neurotransmitter activities suggest neurological dysfunction consistent with prior findings on autonomic-cardiovascular and brain-muscle connections.
Altered Muscular Gene Activities
Similar to the immune profile alterations, ME/CFS patients also exhibited changes in muscle-related gene activities that are sex-dependent.
In males, genes associated with sugar metabolism and mitochondrial functions were downregulated, while those involved in fatty acid breakdown were upregulated, indicating shifts in muscle cell energy processing.
For females, genes related to growth hormone receptor signaling and protein regulation via ubiquitin were upregulated, while those tied to fatty acid oxidation and mitochondrial functions were downregulated. This pattern suggests disruptions in energy processes within muscle cells.
These findings highlight significant disparities in muscle gene expression between male and female ME/CFS patients, reflecting distinct changes in muscle energy metabolism consistent with the impaired brain-muscle connection previously discussed.
Altered Immunological Activities
ME/CFS patients showed elevated levels of PD-1, a marker of T-cell exhaustion, in their cerebrospinal fluid compared to healthy controls. Changes in blood revealed an increase in naïve B-cells but a decrease in memory B-cells among ME/CFS patients.
Sex-based analyses indicated that male patients had heightened expression of certain receptors on T-cells in cerebrospinal fluid, while female patients had a higher presence of naïve T-cells in their blood. Gene expression studies revealed changes in immune signaling genes for males and alterations in B-cell function genes for females.
Overall, these findings underscore the complexity of immune changes associated with T-cells and B-cells in ME/CFS, which differ by sex.
Altered Gut Microbiota Profile
Stool sample analyses indicated that ME/CFS patients had lower diversity in their gut microbiota compared to healthy controls. The overall composition of their gut microbiota—encompassing the types and quantities of bacteria—also differed between the two groups.
Other Observations
No significant differences were observed in lung function, muscle oxygenation, resting energy expenditure, basal mitochondrial function in immune cells, muscle fiber composition, or body composition. These null findings suggest that ME/CFS does not present with a resting low-energy state.
Other null results included the absence of lymph node enlargement, brain lesions, inflammation, small fiber neuropathy, neuronal injury, heavy metal toxicity, hypermobility, mitochondrial function issues, orthostatic hypotension, cell senescence, and autoimmunity.
These findings indicate that these factors are not implicated in ME/CFS pathomechanisms, making them unlikely targets for clinical treatment.
Piecing Together the Findings
Based on their results, the authors of the study proposed a model outlining the disease mechanisms of ME/CFS (Figure 1).
An initial infection triggers immune dysfunction and gut dysbiosis (a negative alteration of gut microbiota), potentially leading to long-lasting effects. These immune and microbiota shifts may impact brain function, resulting in decreased levels of various metabolites related to catecholamine and neurotransmitter metabolism.
This altered biochemical environment can affect several brain structures, including those governing the autonomic nervous system. Consequently, autonomic dysfunction may lead to reduced heart rate variability (HRV) and baroreflex-cardiovagal function, ultimately impairing cardiopulmonary capacity.
Similarly, brain areas that control muscular function, particularly the motor cortex, may also be affected, resulting in diminished physical capacity, especially in maintaining force output. This impairment is compounded by dysfunctions in the autonomic-cardiovascular and cardiopulmonary systems.
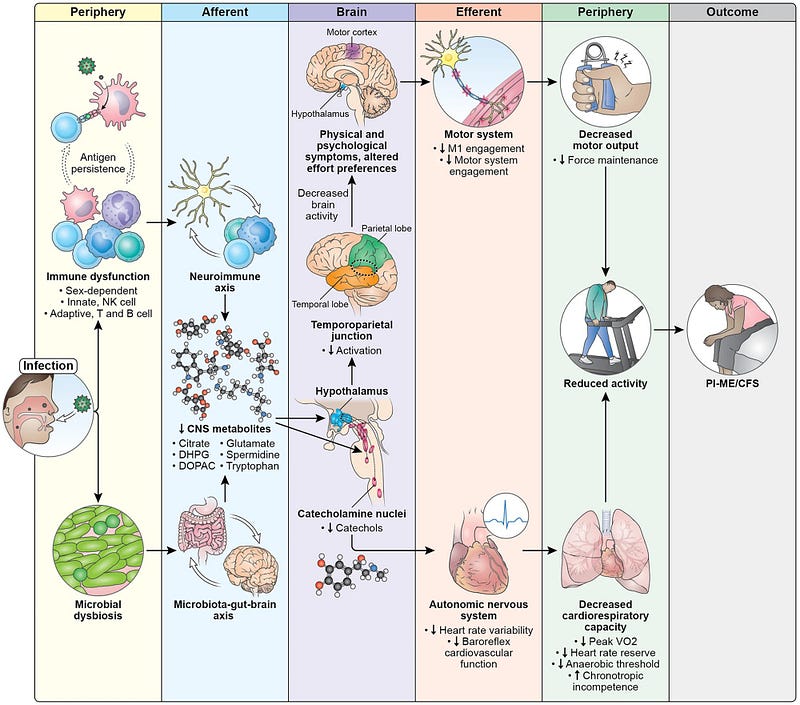
Importantly, the study authors note that this model identifies potential therapeutic intervention points and clarifies why some treatments may not yield results. For example, T-cell exhaustion suggests that immune checkpoint inhibitors could be beneficial by eliminating persistent foreign antigens. Changes in neurochemistry affecting neuronal circuits may also present another avenue for intervention.
However, simply targeting symptoms—through exercise, cognitive behavioral therapy, or autonomic system therapies—might not address the core pathomechanisms of ME/CFS.
“This study provides a more extensive set of biological measurements in individuals with post-infectious ME/CFS than any prior research,” stated Avindra Nath, MD, the clinical director at the NIH who led the study. “It establishes that there is a distinct biological basis for this disease, characterized by clear abnormalities in both immune and neurological systems.”
The Path Forward
According to Neurology Today, other experts regard the NIH study as pivotal, primarily because it stands as the most extensive investigation to date affirming a biological basis for ME/CFS.
Dr. Nath explained, “Throughout the history of neurology, numerous diseases have been challenging to explain, often dismissed as ‘malingering’ or ‘hysteria,’ such as epilepsy and dystonia. As science has advanced, we’ve come to recognize these as genuine brain-driven illnesses that can be treated, and they no longer face dismissal. ME/CFS has encountered similar skepticism.”
As mentioned earlier, ME/CFS has been widely overlooked since its initial documentation in 1955, with long Covid facing similar initial reactions. Experts emphasize that both conditions represent analogous post-infection syndromes, and some long Covid patients have even received ME/CFS diagnoses.
Vicky Whittemore, PhD, director of the NIH neurosciences division, noted, “Even as recently as 2015, when I began overseeing grants on ME/CFS, there remained a perception that it was psychological.” Dr. Whittemore added, “All blood tests, imaging, and other evaluations would return normal results, leaving patients to be told there was nothing wrong and to consult a psychiatrist.” Nonetheless, Dr. Whittemore’s concerns remain. Despite the newly described ME/CFS pathomechanism model, identifying a specific biomarker or clinical test for diagnosing ME/CFS still necessitates further research.
Additionally, it is crucial to acknowledge that this model is not yet proven. The authors themselves recognize that their study is primarily correlational. Establishing cause-and-effect relationships for a condition with such complex mechanisms as ME/CFS poses considerable challenges. It would be unethical to induce these mechanisms in humans to verify their causative roles, so researchers must rely on animal models. Even then, extrapolating findings from animal studies to humans necessitates a significant leap of faith.
One potential path forward involves investigating reverse causation through treatments directed at the identified mechanisms. Based on the proposed model, the authors suggest immune checkpoint inhibitors or neuropsychiatric medications—aimed at correcting immune exhaustion or neurochemical changes in the brain—as possible treatments for ME/CFS. Should these interventions prove effective, they would lend credence to the model's causative implications.
Overall, the NIH researchers have made significant strides in establishing a foundation for future ME/CFS research with their newly articulated pathomechanism model.
In closing, if you have read this far, thank you. You can subscribe to my Medium email list here. If you feel inclined, you may also support me here, and I would greatly appreciate any financial assistance.
I began drafting this article nearly two months ago and finally completed it, thanks to Medium’s Draft Day.